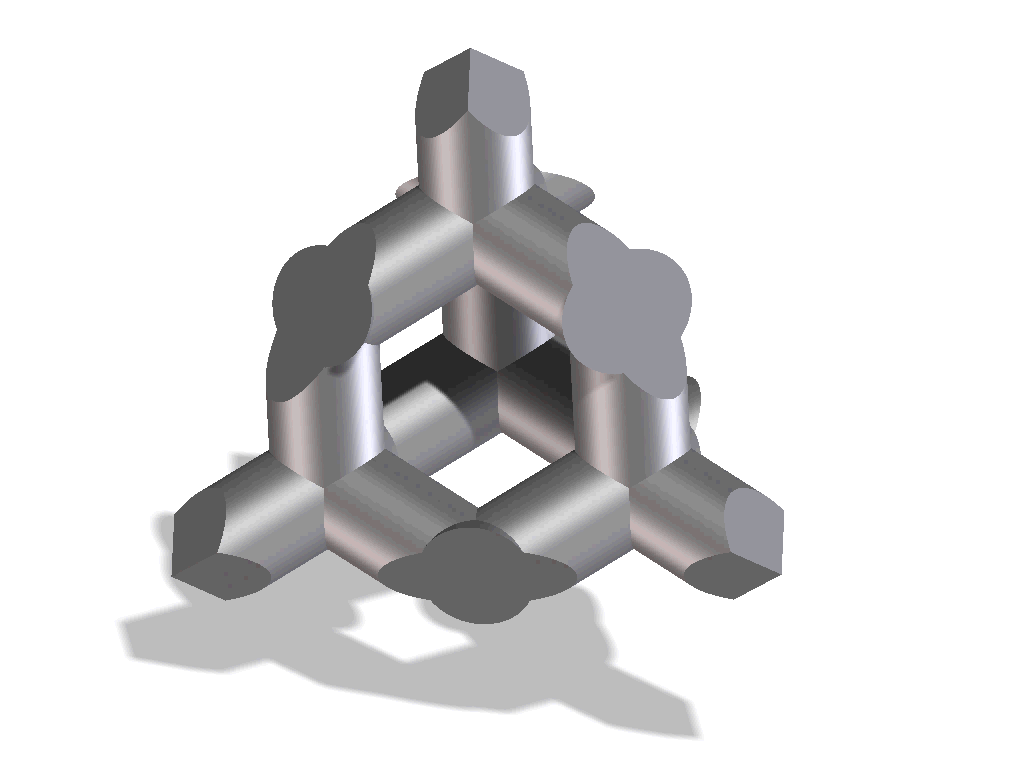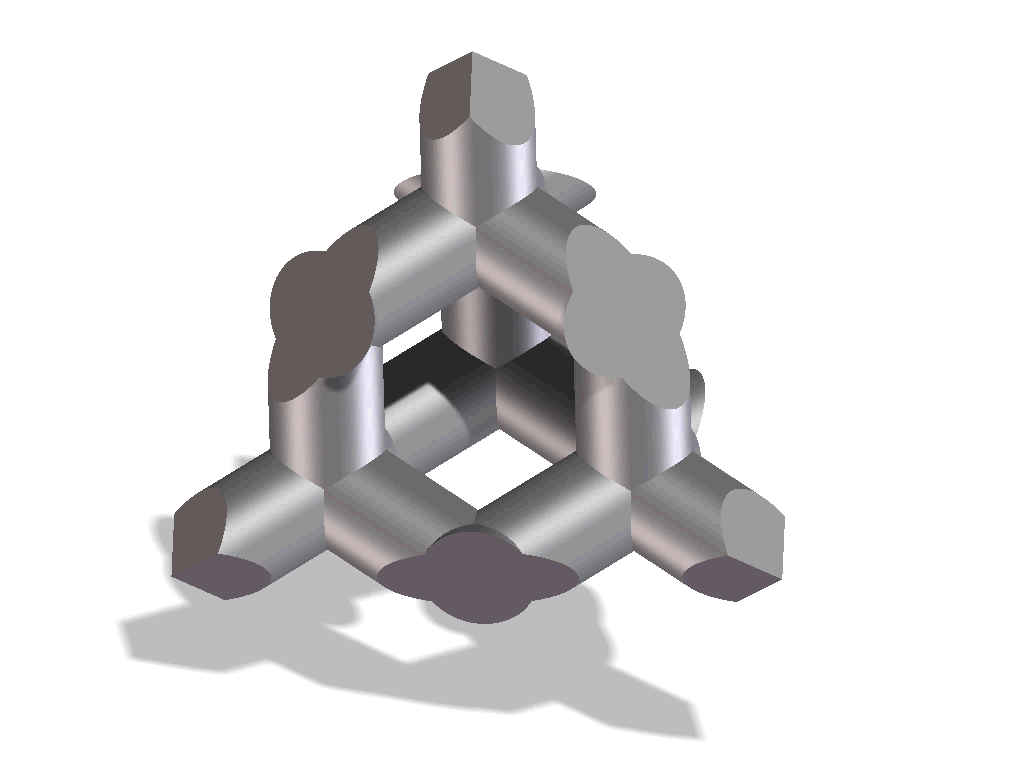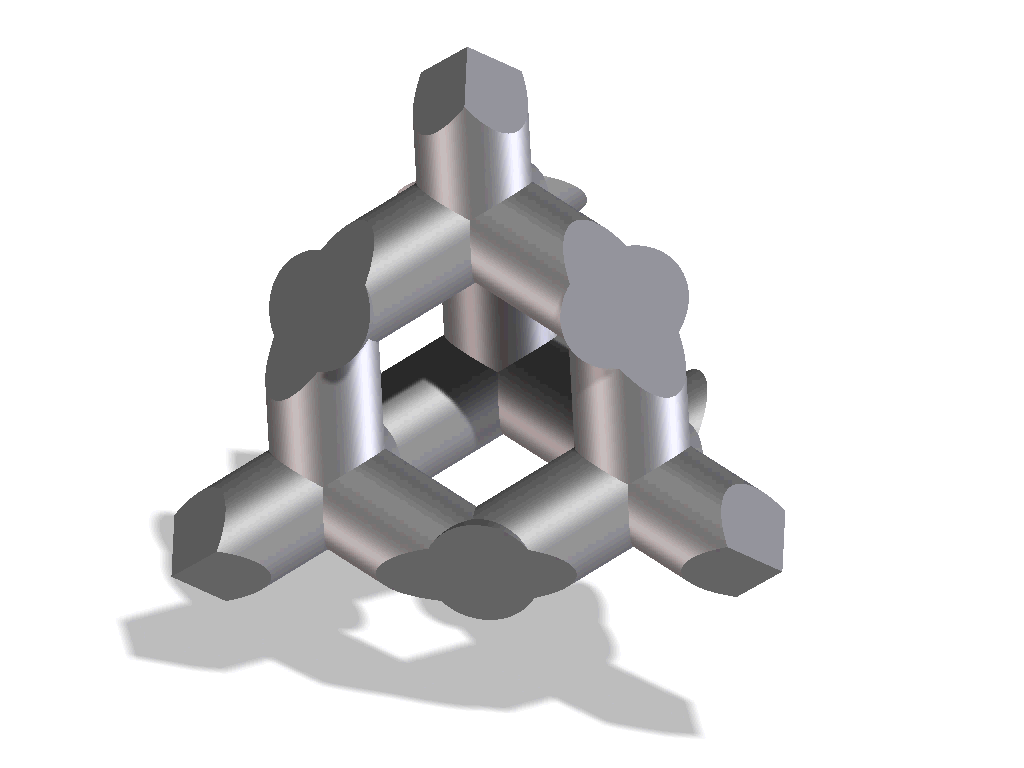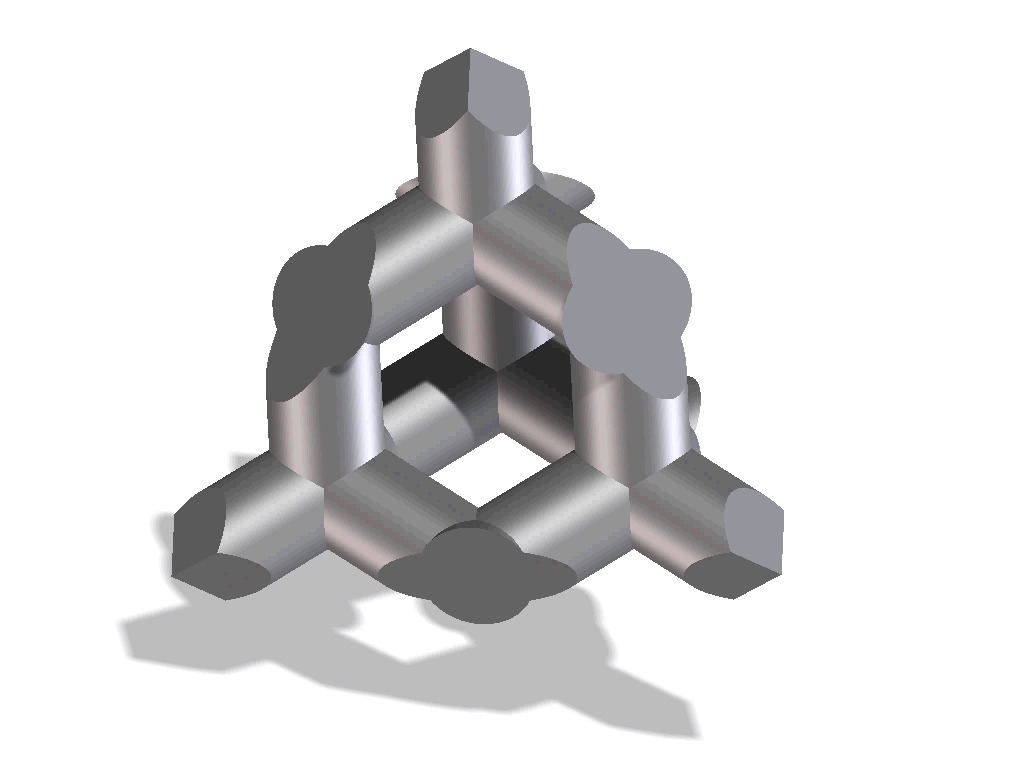
Improved osseointegration in spine surgery with intervertebral fusion implants made of structured titanium
Modern spinal surgery places significant demands in the design, mechanics, and biocompatibility of implants. The effective osseointegration of the implants is of critical importance in these surgeries. Implants made from structural titanium not only satisfy the demands in terms of primary stability, compression stability, and elasticity, but also create all of the conditions which are essential for successful osseointegration. Based on the latest insights regarding the optimization of osseointegration, titanium with an open-pored structure (‘structural titanium’) was developed for use in spinal surgery. In this documentation, we describe
the morphological, mechanical, and biological properties of intervertebral fusion implants made from structural titanium.
In addition to implant biomechanics and biocompatibility, increasing importance is being attached to successful osseointegration in modern spinal surgery. Bony fusion between adjacent vertebral bodies has been the goal of treatment for various spinal disorders. Originally, these techniques required a substantial amount of muscle dissection, extensive use of autologous bone graft, and prolonged bed rest. Spinal instrumentation was introduced to increase the fusion rates while shortening recovery times. This also allows surgeons to better correct spinal deformities. In addition, implementation of anterior column support has led to improved results. Initially, this entailed positioning a bone graft in the intervertebral disc space via an anterior or posterior approach and resulted in
better vascularization of the graft, a more durable fusion, and more effective maintenance of lordosis.
Unfortunately, depending on the indication and bone quality, the use of interbody bone graft alone was often not enough to achieve adequate stability. Furthermore, harvesting of autologous bone graft from the iliac crest, for example, frequently resulted in postoperative pain and complications. For these reasons, and to improve fusion rates, intervertebral ‘cages’ or ‘spacers’ were developed. Since that time, intervertebral fusion cages have become firmly established as the standard of care for interbody fusion.
Biomechanics, biocompatibility and osseointegration
The osseointegration of the fusion cage has proven to be of major importance. Various materials have been tested to determine their biocompatibility with
varied results. Today, most cages are made from titanium or polyetheretherketone (PEEK) and are hollow, allowing them to be filled with bone graft (autograft, allograft) or a graft substitute. It is well documented that titanium promotes osseointegration when it has an open-pored structure and thus it holds the promise of improved treatment outcomes. Here, a distinction needs to be made between an implant that has a contact surface that is microscopically or macroscopically rough versus one with a trabecula-like structure with interconnecting pores. Whereas microscopic or macroscopic roughness increases the contact area, an open, porous structure can also serve as a ‘scaffold’ and make it possible for blood vessels and bone cells to penetrate deep into the implant.
Titanium, the biocompatible material - a short material science
Titanium is a biologically inert and biocompatible material and has been used in human and veterinary medicine since the 1930s. Its mechanical and
biocompatible characteristics have proven ideal for this purpose that time. This is due firstly to the fact that titanium exhibits a high degree of stability,
particularly in terms of contact with body tissues and when under load. When in contact with oxygen, titanium forms a protective oxide film which is extremely adherent, insoluble, and chemically stable, thus making it highly resistant to corrosion in a septic environment. In addition, this makes titanium "invisible" to the body, causing no defensive response from the immune system.
Secondly, its high dielectric constants permit titanium to form a permanent physical connection with the surrounding bone tissue (osseointegration) due
to the fact that it allows bone cells to grow on the contacting surface. Compared with other biocompatible materials such as stainless steel, titanium is many times lighter and nonetheless possesses a higher tensile strength, thus permitting an ideal strength-to-weight ratio for medical use. Not least, titanium is highly machinable and its outstanding properties can be enhanced even further using new process and surface technologies.

The open, porous structure of the ST implants increases the area which can be colonized by new bone cells, hence increasing the contact surface between the implant and the endplate of the adjacent vertebral body. This, in conjunction with the surface roughness of the implant, results in a high degree of primary stability and significantly reduces the risk of migration. This is also aided by the open and interconnecting porosity, which makes it possible for autologous cell material to penetrate and spread within the implant.
The completely interconnected three dimensional pore structure of the ST implants is similar in macroscopic terms to the structure of cancellous bone. This promotes rapid vascularization and osseointegration. Interconnectivity of the pores and the appropriate pore size also result in new bone growing further than just the implant’s external surface, but rather deep into its structure. Thus, a structural connection is established between the implant and the bone which may help to create increased segmental stability.
One important requirement on porous titanium is the maintenance of the predefined, favorable pore geometry and three-dimensional structure. Manufacturing processes such as powder sintering, combustion synthesis and ‘space-holder’ methods or even Electron Beam Melting (EBM) have proven less capable in this regards. In contrast, the manufacturing process Direct Metal Laser Sintering (DMLS) makes it possible to produce implants with complex predefined internal and external structures
There are two morphological requirements for modern fusion implants in clinical use. First, it must be possible to insert the implants in a manner so as to maximize tissue preservation and avoid trauma during implantation. This is ensured by the smooth lateral surfaces of the ST implants. Second, it must be feasible using standard diagnostic imaging techniques to reliably detect whether or not fusion has occurred. In this respect PEEK implants have had an advantage and this was a key factor in this material’s breakthrough into spinal surgery. Implants made from structural titanium also fulfil this requirement as their open structure and low total volume of the titanium permit them to be imaged without any obstructive artefactsIn clinical application there are two further morphological requirements for fusion implants. On the one hand, implants must be able to be positioned as tissue-conserving and atraumatic as possible during insertion. In the case of the ST-Line implants, this is ensured by the smooth lateral surfaces.
Despite the open structure and low total volume of titanium, ST implants exhibit a high level of mechanical strength. For example, the dynamic compression stability of the MOBIS II ST implant is 5.2 times higher than that of a comparable commercially available, Food and Drug Administration (FDA) approved PEEK cage. Clinical studies have also shown that porous titanium implants are very efficacious and safe.
It has also been shown that ST implants not only have high degrees of primary stability and mechanical strength but also exhibit an elastic modulus that is similar to bone. Standard titanium implants may exhibit what is known as a stress shielding effect. This may result in the resorption of the surrounding bone tissue along with inhibited fusion resulting from the formation of connective tissue. However, this risk is minimized with ST implants due to their low elastic modulus.
The DMLS production process can be used to generate predefined pore geometries (size, depth, and interconnectivity) which simulate the three dimensional
structure and mechanical properties of natural bone. Furthermore, it has been shown in in vitro experiments that the metabolic activity of osteoblasts
applied to and cultured on structural titanium remains very high even after several days (Figure 6). In vivo animal studies have also shown that porous titanium material has a positive effect on the regeneration of bone defects and the colonization of new bone cells. It can therefore be assumed that structural titanium promotes the penetration of bone cells into the implant structure and thus forms the basis for rapid and solid fusion.
Microscopic histological image of the cross section of an structured titanium implant removed from an ovine model after 12 weeks. In the space within the diamond grid structure of the implant, there is evidence of the formation of a large area of spongiosa bone structure, extending from the central area of the cross section to the superior margin. (Histological findings from a formalin-fixed titanium lattice cage removed from an ovine model; Foundation: Hannover Veterinary Medicine School)

In terms of mechanical strength ST implants are at least as strong as the conventional fusion implants and their elastic modulus is closer to that of bone than that of standard titanium implants. As a result the risk of stress shielding (which inhibits osseointegration) can be minimized. Implants from the ST line can be inserted in a manner so as to preserve tissue and minimize the risk of neural injury while at the same time they can be effectively imaged by x-ray or CT. Clinical studies also show that porous titanium implants exhibit high levels of efficacy and safety.
Conclusion
In developing implants made from structural titanium, SIGNUS Medizintechnik is drawing on the latest insights on the optimization of osseointegration in
endoprosthetic implants and is implementing these in the field of spinal surgery. ST implants possess interconnected pores with a diamond grid structure
that permits the growth of bone onto and into the implant and thus creates the platform for a stable intervertebral fusion.