TETRIS II
Request product
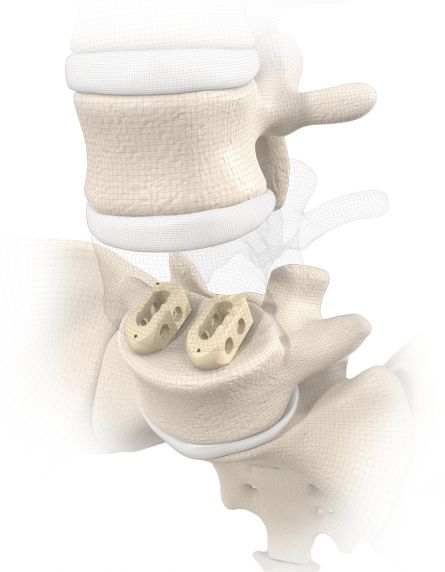
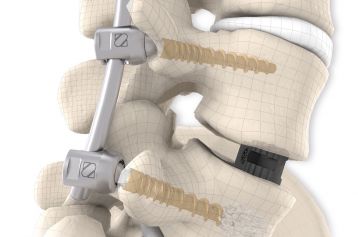
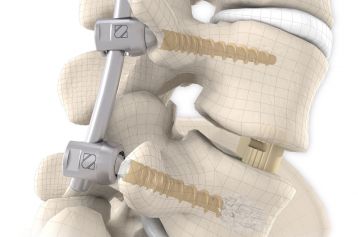
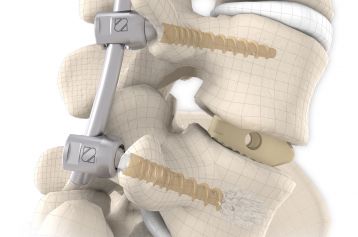
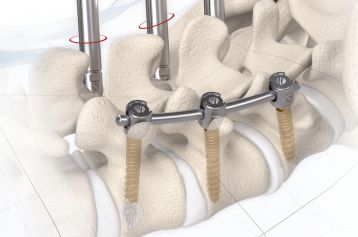
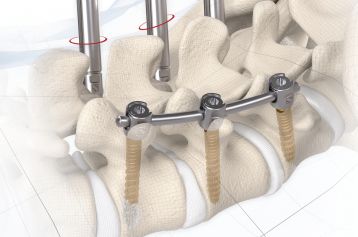
start requestPosterior lumbar interbody fusion (PLIF) is today the gold standard in the treatment of degenerative disc disease. The TETRIS™ Lumbar Fusion Implant System is designed to provide the surgeon with an optimal method for achieving immediate and long-term biomechanical stabilisation and restoration of lordosis in the lumbar spine (L1 to S1). The implants are inserted bilaterally in pairs via a posterior approach.
They are intended to restore the interbody vertebral disc height and lead to solid bony fusion when used in conjunction with supplemental posterior instrumentation of the surgeon’s choice.
TETRIS™ II is placed by a PLIF (Posterior Lumbar Interbody Fusion) approach in the L1 – S1 spinal region and should be inserted in pairs. The large fenestration in the implant permits the cage to be packed with natural or synthetic bone graft substitute such as KAINOS® Inject.
The inserted cages, combined with additional posterior instrumentation, lead to immediate biomechanical stabilisation. This establishes the ideal conditions for vertebral body fusion.
The large selection of implants provides for a high degree of intra-operative flexibility and ensures restoration of the intervertebral space. In addition to plane-parallel implants, the TETRIS™ II cage is also available with a 4° lordotic angle.
Placement in the gold-standard PLIF technique
Open implant design
Flattened implant apex
Smooth lateral surfaces
Proven SIGNUS toothed cage design
Tantalum markers
PEEK-OPTIMA® is a biocompatible polymer offering a number of benefits in this indication. In its strength it is comparable to cortical bone and due to its excellent MRI compatibility permits artifact-free follow-up examinations.
The tantalum markers are used with x-ray or CT during and after surgery to check that the implant is correctly positioned.
Here you get to the eifu of TETRIS II.