MOBIS II
Request product
start request
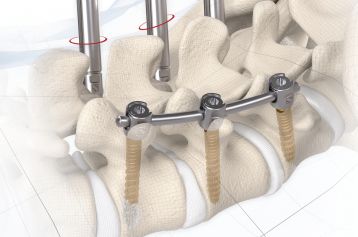
Intersomatic fusion via the transforaminal approach is largely consistent with the principle of PLIF (Posterior Lumbar Interbody Fusion). The transforaminal (TLIF) approach entails unilateral resection of the joint. This enables convenient access to the disc whilst at the same time preserving the contralateral lamina and facet joint as an additional fusion surface.
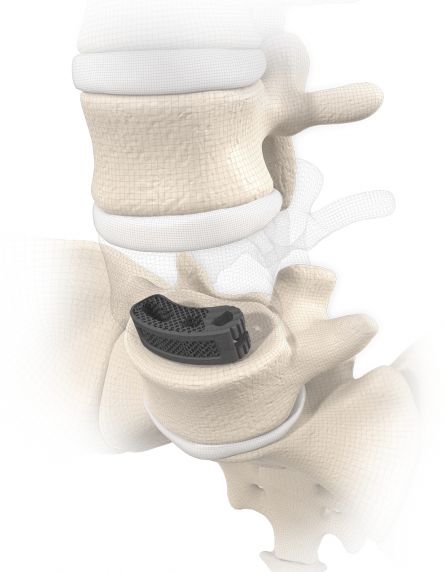
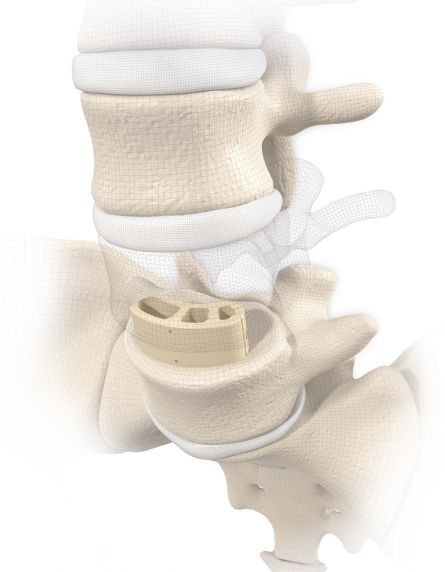
MOBIS® II ST is placed by a TLIF (transforaminal lumbar interbody fusion) approach in the L1-S1 spinal region. Its arc-like profile with large hollow openings guarantees a large contact surface with the bone. With its shape MOBIS® II is an ideal fit to the anterior curve of the vertebral body.
The large fenestration in the implant permits the cage to be packed with natural or synthetic bone graft substitute such as KAINOS® Inject.
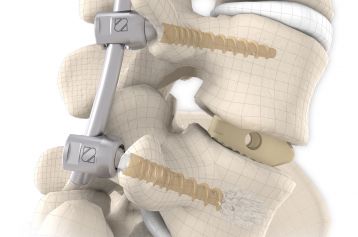
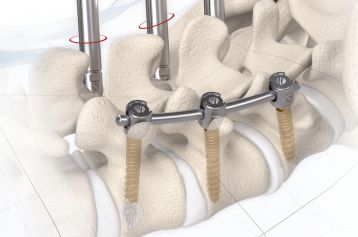
The inserted cage, combined with additional posterior instrumentation, leads to immediate biomechanical stabilisation. This establishes the ideal conditions for vertebral body fusion.
The large selection of implants provides for a high degree of intraoperative flexibility and ensures restoration of the intervertebral space. In addition to plane-parallel implants, the MOBIS® II ST cage is also available with a 5° lordotic angle.
Controlled variable insertion
Open implant design
Flattened implant apex
Smooth lateral surfaces
Increased roughness* in conjunction with proven SIGNUS toothed cages design
Open, macroporous titanium structure*
Tantalum markers*
MOBIS II PEEK: PEEK-OPTIMA® is a biocompatible polymer offering a number of benefits in this indication. In its strength it is comparable to cortical bone and due to its excellent MRI compatibility permits artifact-free follow-up examinations.
The tantalum markers are used with x-ray or CT during and after surgery to check that the implant is correctly positioned.
MOBIS II ST: Manufactured from a biocompatible titanium alloy (TiAl6V4) with proven strength.
The open, porous structure of ST implants increases the surface area for new bone cells to settle, thus increasing the contact surface between the implant and the end plate of the adjacent vertebral body. In combination with the surface roughness of the implant, a high primary stability and a significantly reduced risk of migration can therefore be achieved. This is additionally favoured by the open and interconnecting porosity, which enables successful grafting of endogenous cell material and its propagation.
Click here for detailed ST material information.
Here you get to the eifu of MOBIS II.