RABEA
Request product
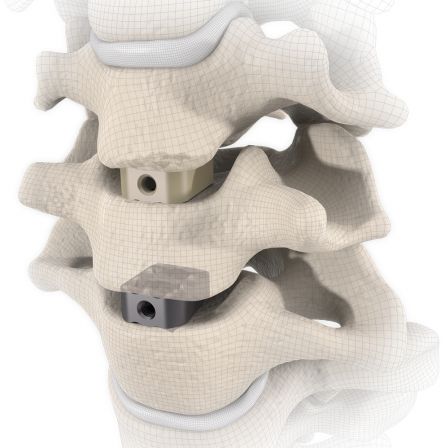
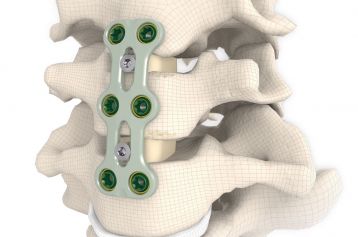
start requestThe RABEA® disc replacement implant was developed for the specific requirements of anterior cervical vertebral fusion (C3 – TH1). The implant ranges are available in various designs, footprints, heights and angles to enable adaptation to different patient anatomies.
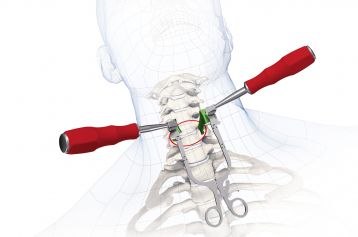
RABEA® is implanted in the C3 – TH1 cervical spinal region using the proven Smith-Robinson technique.
The open design of the implant permits the cage to be packed with natural or synthetic bone graft substitute such as KAINOS® Inject.
The large selection of implants provides for a high degree of intraoperative flexibility and ensures restoration of the intervertebral space. For RABEA® there are cages available in a 5° lordotic angle as well as plane-parallel implants.
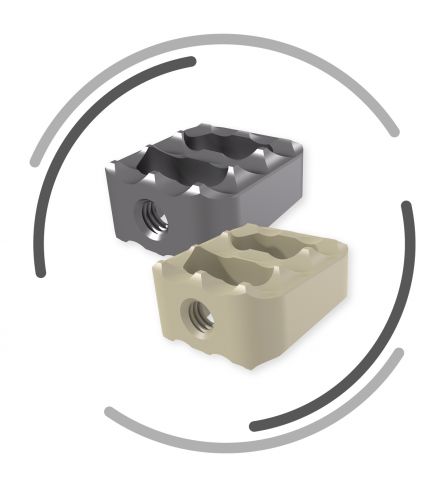
Rectangular design
Open implant design
Artefact–free MRI imaging*
Proven SIGNUS serrated cage design
Titanium marker*
Only one instrument required for implantation
The implants are made either of titanium aluminium vanadium (Ti-6Al-4V) or the high-performance medical polymer PEEK-OPTIMA®. The radiolucent PEEK-OPTIMA® implants have anterior and posterior X-ray markers made of titanium alloy (Ti-6Al-4V) to enable intraoperative and post- operative verification.
Here you get to the eifu of RABEA.