NEVIO
Request product
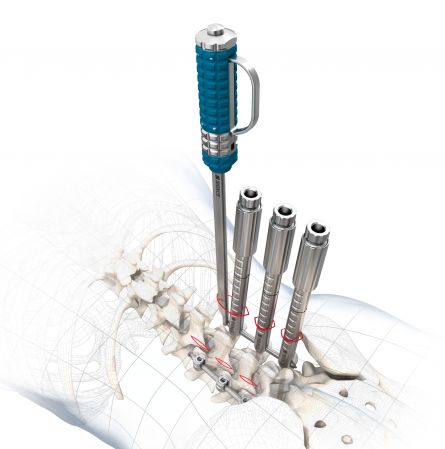
start requestThe NEVIO System in combination with the MONOPOLY rod screw system is intended for use on the thoracic/lumbar spine (Th2 - L2) and is used for internal minimally invasive dorsal stabilisation.
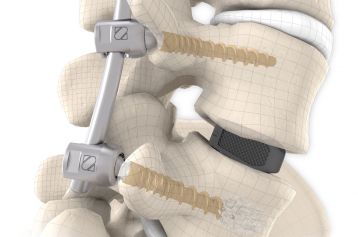
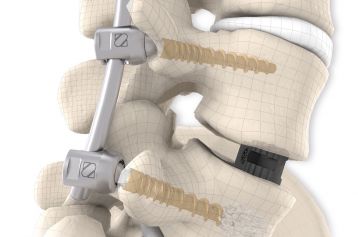
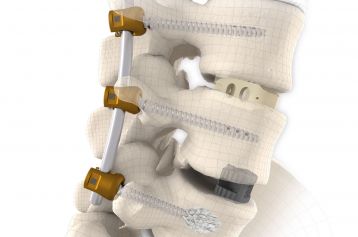
The system is used in one or more segments until the bony fusion of the spinal column has taken place. NEVIO in combination with MONOPOLY offers a sufficient selection of components with well thought-out design for this system.
The NEVIO rods are available in different lengths, straight and pre-contoured. The cannulated pedicle screws of the MONOPOLY system are used for screw implantation. The MONOPOLY screws are not modular and are only available as one-piece implants.
Further information on this topic can be found in the chapter MONOPOLY.
Highly stable "towers" that allow safe percutaneous instrumentation
The use of existing cannulated MONOPOLY screws ensures easy handling
Stable guide wires of 1.8 mm thickness allow safe screw guidance
The persuader integrated in the towers simplifies the setting of the rod and enables reduction maneuvers
The implants consist of the following materials:
- titanium alloy (Ti-6Al-4V) according to ASTM F 136 / ISO 5832-3
- Cobalt-chromium-molybdenum alloy according to ASTM F 1537 / ISO 5832-12:
Composition:
Titanium alloy (TiAl6V4) according to ASTM F 136 / ISO 5832-3.
For all titanium alloy TiAl6V4 products:
Nickel free according to ASTM F 136 / ISO 5832-3
Nitrogen 0,05% max, carbon 0,08% max, hydrogen 0,012% max, iron 0,25% max, oxygen 0,13% max, aluminium 5,5-6,5%, vanadium 3,5-4,5%, balance titanium.
Cobalt-chromium-molybdenum alloy according to ASTM F 1537 / ISO 5832-12:
Carbon 0.014% max, chromium 30.0% max, molybdenum 7.0% max, nickel 1.0% max, iron 0.75% max, silicon 1.0% max, manganese 1.0% max, nitrogen 0.25% max, balance cobalt.
For easy identification, the implants are coated with differently coloured oxide layers. Changes in colour are due to production and preparation and have no influence on functionality.
The materials are established for use as implants. They are biocompatible, corrosion-resistant, non-toxic in the biological environment and allow for trouble-free imaging in X-rays.
Here you get to the eifu of NEVIO.