DIANA
Request product
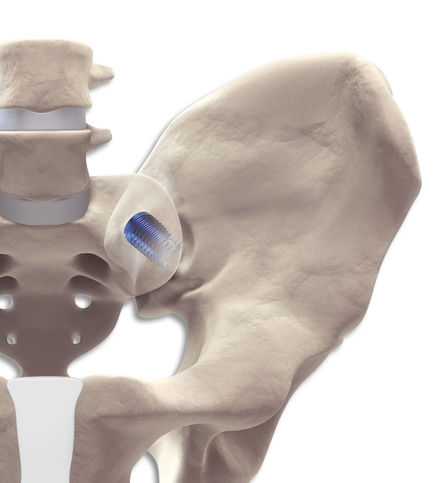
start requestThe sacroiliac joint (SIJ, also known as the iliosacral joint) is a loadbearing joint between the ilium and the sacrum. It is a true joint that has a subchondral articular surface, joint cartilage and a ligamentous apparatus. Although the SIJ makes only minor translational and rotational movements, over the course of a lifetime it can develop signs of wear. The reason for this is its position in the weight-bearing axis where it is exposed to large compression and shearing forces.
DIANA® provides a simple and safe treatment technique for the sacroiliac joint.
DIANA® – The name of the procedure is based on its underlying concept:
Distraction-Interference-Arthrodesis sparing Neurovascular (nerve and blood vessel related) Aspects.
In a gentle and low-risk procedure, the DIANA® method anchors an interference implant via a posterior access distally in the iliac bone. The proximal and central implant area is located in the extra-articular recess without affecting the actual articular surfaces. The surgical access to the recess and the path of the instruments and the implant follow the iliac diaphysis and thus the weight-bearing axis of the pelvis. The intraoperative radiographic images are adjusted taking this axis into consideration. The transition of the bony segments of S1 and S2 thus forms the entry point for the guide wire and the instruments, the trajectory of which travels from this point towards the hips. The joint space is then distracted during the instrumentation, relieving stress on the joint cartilage as well as capsular neural structures and those neural structures immediately ventral to the joint. It is thus possible to approximate the original state of the pelvic girdle using ligamentotaxis. The entire procedure is carried out within a safe access zone with no weakening of the musculature or the bony structures. Retraction and revision are still entirely possible.
Targeted, reproducible placement of the DIANA® implant is achieved with sequential instrumentation. The distraction and ligamentotaxis performed with the instrument set stabilises the joint during the fusion process. The unique and nevertheless intuitive to use instrument set operates exclusively in the ‘safe zone’ and thus avoids many of the complications associated with traditional SIJ fusion techniques. The risk of damaging nerves and vessels is thus minimised. Together with the colour-coded implants and guide instruments, the step-by-step and intuitive implantation technique shortens the learning curve. Unlike other techniques, DIANA® a llows t he p reparation o f t he e xtraarticular recess with generous apposition of bone mass: another requirement for successful fusion and a reduction in the rate of complications.
The implant design has been optimised for the application technique and mechanically safeguards the stability during the fusion process.
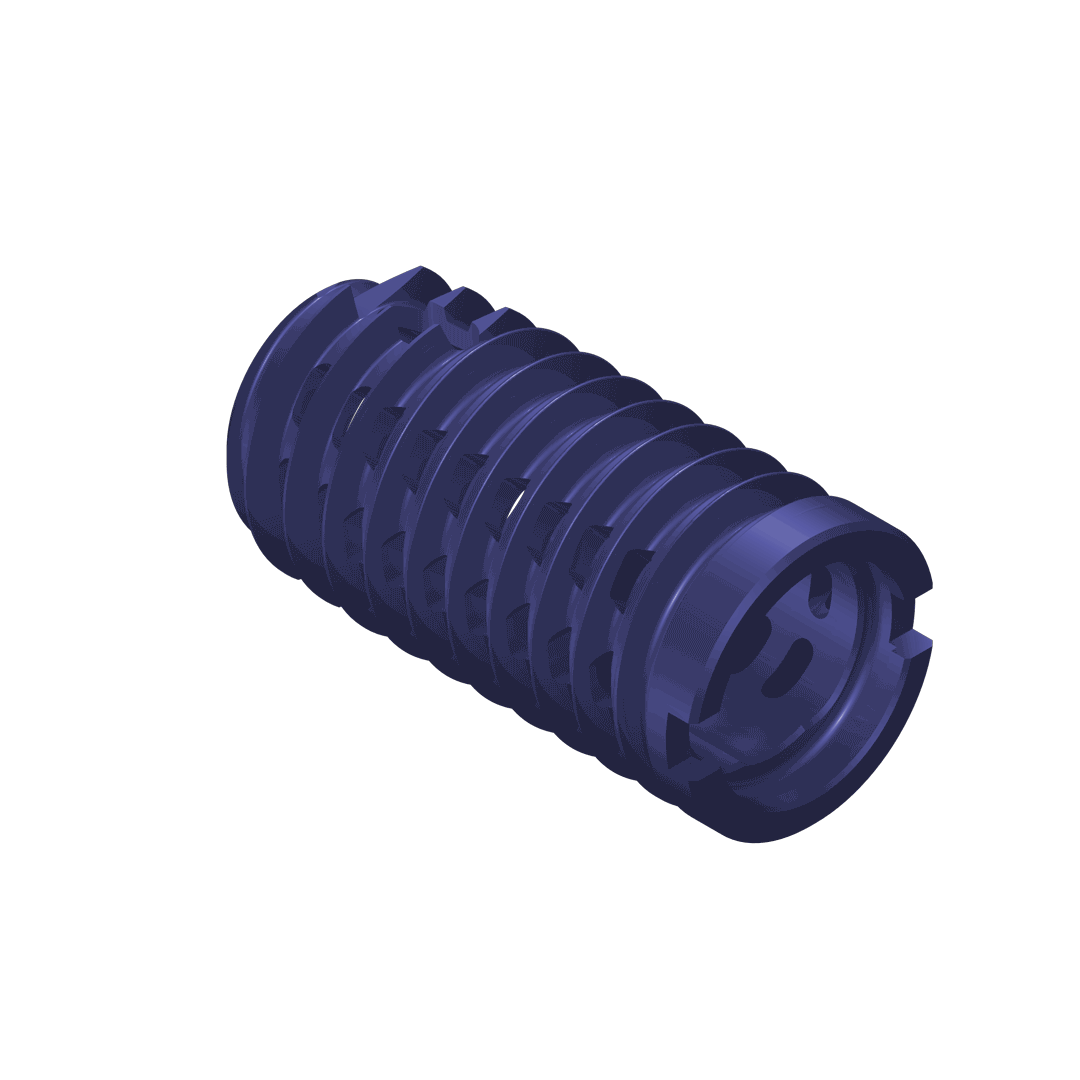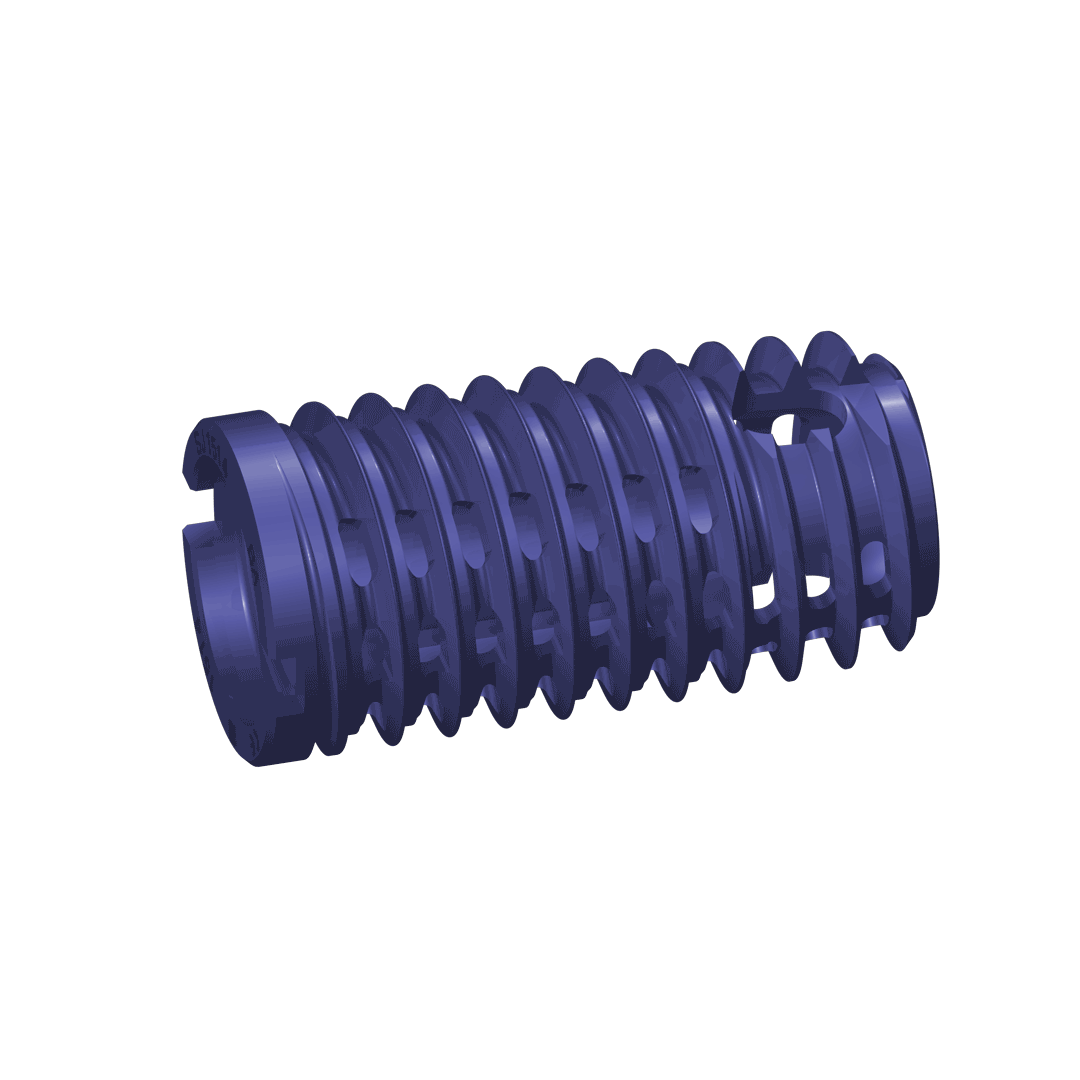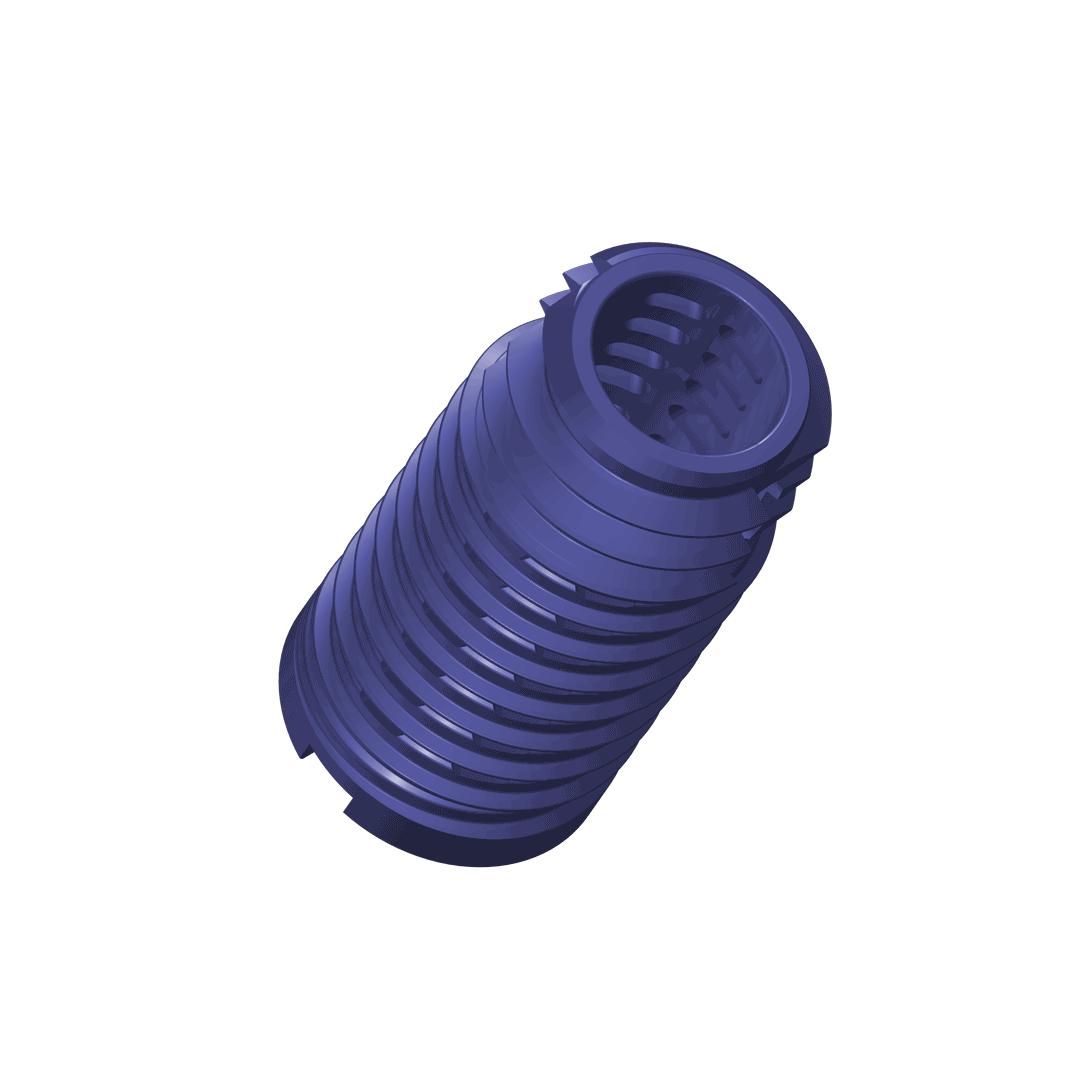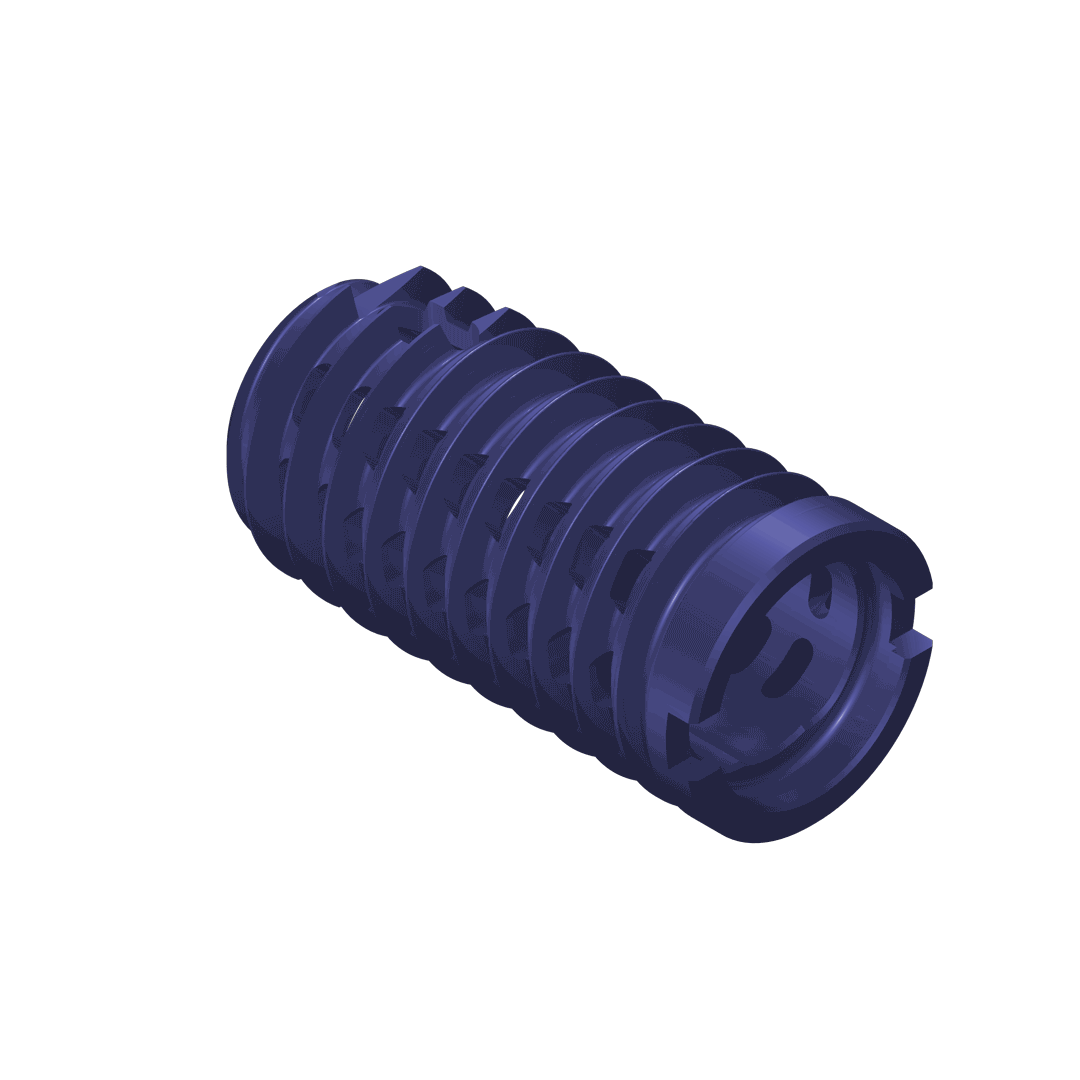
The DIANA® implant has a conical design to optimise stability. The selftapping multifunction thread ensures distal fixation in the iliac bone and the broad supporting area (thread shoulders) offers protection against subsidence as well as better bone hold. For adaptation to the particular anatomical situation, implants with different diameters are available.
The implant
The instrument set
The procedure
Material specification: Manufactured from a biocompatible titanium alloy (Ti-6Al-4V) with proven strength.
Here you get to the eifu of DIANA.